Changing Lives, One Patient at a Time
QOL Medical
QOL Medical is a specialty biopharmaceutical company dedicated to improving each patient’s life. We focus on solving complex, rare disease challenges with elegant solutions and want to change the world, one patient at a time.
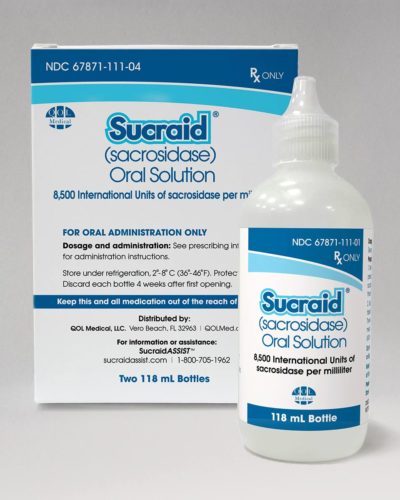
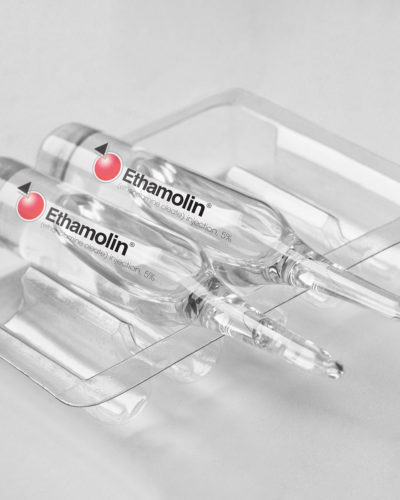
QOL Medical was founded in 2003 to focus on the commercialization of orphan products for underserved markets. We provide expanded clinical awareness and patient access for the treatment of rare and orphan diseases. The company currently markets two FDA-approved products: Sucraid® (sacrosidase) Oral Solution and Ethamolin® (ethanolamine oleate) Injection, 5%.